NP001 is an investigational immunotherapy for the treatment of ALS that has not been approved by the US Food and Drug Administration.
The safety and efficacy of NP001 in this indication have not been established. Statements and contents on this website have not been evaluated by the FDA or other health authorities.
Proprietary Platform with Transformational Potential
NP001 is an immunotherapy under investigation for ALS engineered for appropriate administration as an infusion into the bloodstream.
This formulation is VERY important for two critical reasons:
- The product is pure enough to eliminate the risk of life-threatening contaminants
- Ability to penetrate deep within innate immune cells to facilitate the formation of an essential regulator of inflammation
NP001 is a small molecule prodrug that is intracellularly converted to taurine chloramine (TauCl) – an established regulator needed to balance pro- and anti-inflammatory processes of the innate immune system. In ALS, TauCl is depleted due to a constant call for inflammatory mediators that results in an imbalance of the innate immune response and destructive inflammation to motor neurons.
By refueling the body with an essential regulator of inflammation, NP001’s goal is to transform tissue-damaging inflammatory macrophages to a non-inflammatory, wound-healing state.
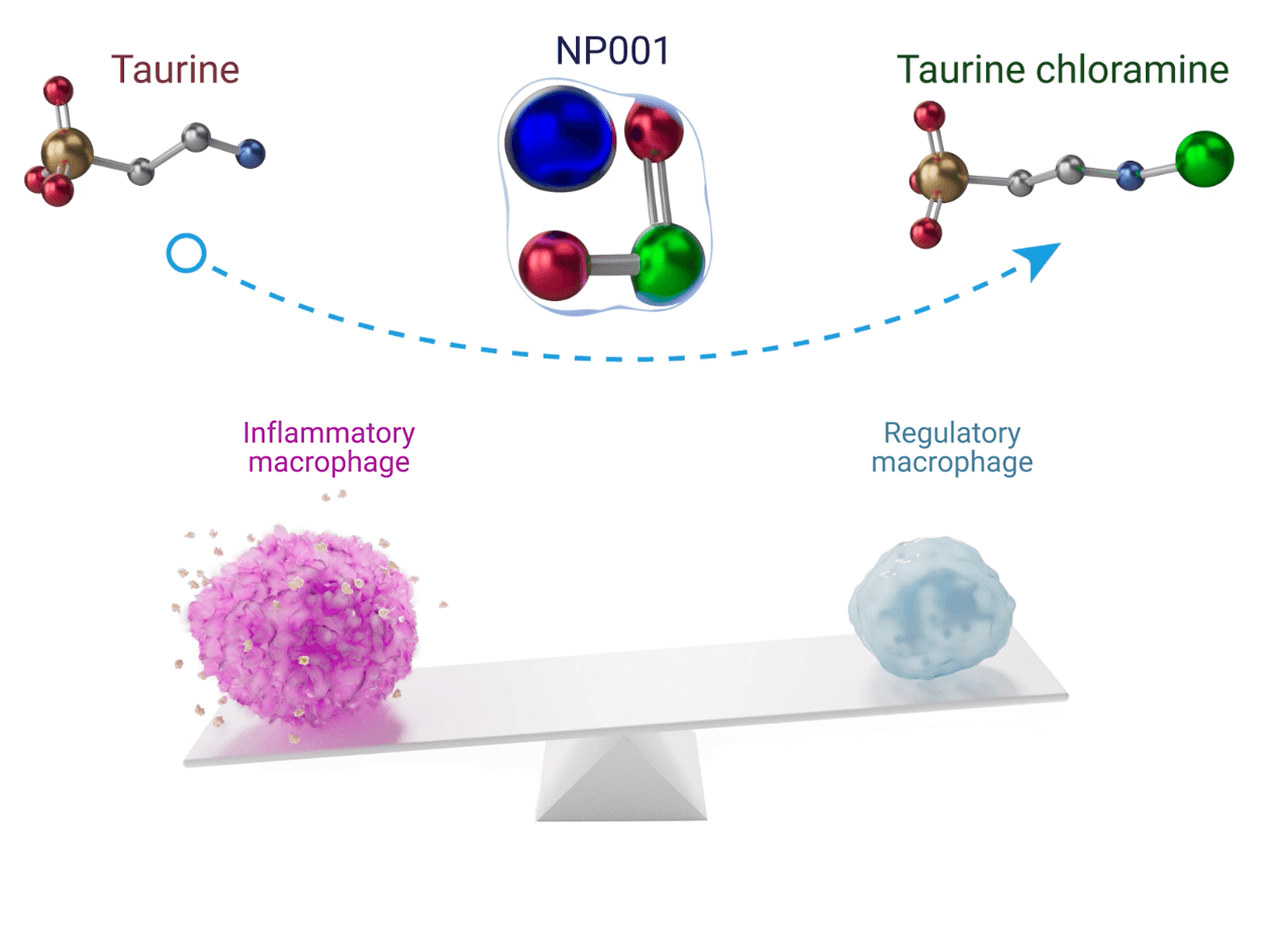
- NP001 is a prodrug to taurine chloramine (TauCl) – an established regulator of innate inflammation
- Taurine is converted to TauCl and released from activated neutrophils to regulate production of inflammatory mediators
- TauCl triggers the resolution of inflammatory processes within macrophages that transform from damaging to wound-healing thus slowing destructive disease processes in patients with ALS